What is the interest of micellar caseins in sports?
Performance improvement is always associated with muscular development. And for this, there is no secret: a good training and an adequate nutrition are essential.
As far as the diet is concerned, it must provide sufficient amount of proteins because these are fundamental building blocks for muscles. For practical reasons, it is often more interesting for athletes to consume protein supplements. And micellar caseins can truly represent a high-quality product! Let this article convinces you!
Micellar caseins? What is it?
Milk contains two types of proteins: soluble proteins (commonly named “whey protein”) and micellar caseins. They respectively represent around 20% and 80% of milk proteins1.
Composition-wise, these proteins are quite similar: the essential amino acidsA contents are very close, like the amount of BCAAB (table 1). Those have a positive effect on the protein metabolism: they promote muscular protein synthesis and reduce breakdown2 3. Leucine is the most important BCAA to stimulate this synthesis. According to the 2018 ISSN (International Society of Sports Nutrition) report on sport nutrition recommendations, the ideal dose of Leucine in order to take advantage of these anabolic effects is likely to be somewhere between 1.7 and 3.5g 2 4.
The main difference between these two protein groups is mainly the digestion speed. Whey proteins are quickly digested and are totally absorbed in about 3 hours. Meanwhile, the assimilation of micellar casein is more progressive and will take up to 7 hours5.
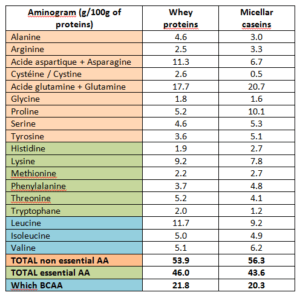
Table n°1: Aminogram of whey proteins and micellar caseins 6 7
But after my workout I want fast proteins! Why would caseins be interesting for me?
“Fast proteins” don’t have only advantages. Yes they are quickly absorbed; on the other hand the blood amino acids concentration suddenly decreases after a few hours. It decreases to such an extent that it reaches lower levels than the initial concentration before protein ingestion. (baseline in figure 1)5.
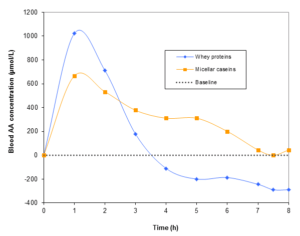
Figure 1: Blood amino acids concentration as a function of time.
In this study5, healthy persons have consumed either a dose of whey (Δ) or micellar casein (O). Blood amino acids concentration was then measured during 8 hours. Baseline: it corresponds to the basal amino acids concentration, i.e. the concentration before protein consumption.
When the blood amino acids concentration is higher than the baseline, muscular protein synthesis is stimulated. However, when it is below, protein breakdown will be promoted…
Although I was told that caseins are anti-breakdown proteins, are they also anabolic proteins? This could be a reason to consume it before going to sleep?
That’s true, this is an excellent product to take just before sleep to have a good amino acids supply throughout the night! Because these proteins are effectively well absorbed and assimilated by the body even if you are sleeping8.
However, there truly is an effect on the muscular protein synthesis. And this has been demonstrated during a 2012 study in which 15 healthy men have taken either caseins or a placebo before sleep. During the night, muscular protein synthesis and breakdown were measured. From these measurements, we discovered that caseins did not really have an effect on protein breakdown, while they did promote synthesis8 9.
Moreover, there is another interesting reason to take caseins before sleep: different studies, especially one conducted on professional soccer players, have proven that caseins enable not only performances and recovery improvement (by muscular protein synthesis promotion), but also muscular soreness reduction10 11.
Ok, but since caseins are slowly absorbed, does it contribute as well as whey protein to the muscular protein synthesis?
Yes they do! A study conducted in 2011 has measured muscular protein synthesis in 17 healthy men. Subjects have consumed either whey proteins, or caseins, or water (control group) after a resistance type exercise. After a 6 hours period, the results showed that caseins stimulate muscular protein synthesis with the same efficacy as whey proteins12!
Other studies have compared the effects of whey proteins and caseins on performances and body composition; and they have demonstrated that there is no significant difference between these two proteins13.
Both proteins stimulate muscular protein synthesis in the same manner, but whey proteins seem to promote protein breakdown after a few hours… In this situation, caseins seem to be more interesting, aren’t they?
Indeed, that is the question we may ask ourselves. If we have a look at the global protein balance, we may venture the hypothesis that caseins might be more interesting than whey protein (figure 2)5.
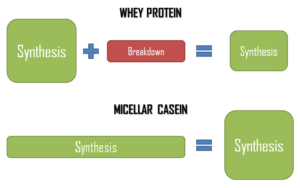
Figure 2: Global protein balance prediction obtains with whey protein and micellar caseins consumption5.
On the market, we can find caseinates. These proteins are obtained by chemical process, whereas micellar caseins are produced through a non-denaturing process. This allows the native structure of proteins to be preserved. At the moment, Ingredia is conducting studies in order to better characterize and compare these two proteins.
For more information, please contact us
Authors: Rémi Maleterre & Audrey Boulier.
A [Essential Amino Acids]: Amino acid that the body cannot synthesize. Therefore they must be supplied by the diet.
*B [BCAA, Branched-Chain Amino Acids]: Essential amino acids which correspond to Leucine, Isoleucine and Valine
______________________________________________________________________________
[1] Swaisgood H.E. (1982). Chemistry of milk protein. Fox P.F. (e.): Developments in Dairy Chemistry. Elsevier Applied Science Publishers, London, UK, 1-59.
[2] Kerksick C.M., Wilborn C.D., Roberts M.D., Smith-Ryan A., Kleiner S.M., Jäger R., Collins R., Cooke M., Davis J.N., Galvan E., Greenwood M., Lowery L.M., Wildman R., Antonio J. and Kreider R.B. (2018). ISSN exercise & sports nutrition review update: research & recommandations. Journal of the International Society of Sports Nutrition, 15:38. Epub August 01, 2018. https://doi.org/10.1186/s12970-018-0242-y
[3] Imanipour V., Banaiifar A., Mahdi F., Naderi A., Sadeghi M. (2018). The effects of branch-chain amino acids on fatigue in the athletes. Interventional Medicine and Applied Science 2061-5094. Epub March 19, 2018. https://doi.org/10.1556/1646.10.2018.10
[4] Jager R., Kerksick C.M., Campbell B.I., Cribb P.J., Wells S.D., Skwiat T.M., Purpura M., Ziegenfuss T.N., Ferrando A.A., Arent S.M., Smith-Ryan A.E., Stout J.R., Arciero P.J., Ormsbee M.J., Taylor L.W., Wilborn C.D., Kalman D.S., Kreider R.B., Willoughby D.S., Hoffman J.R., Krzykowski J.L., Antonio J. International society of sports nutrition position stand: protein and exercise. J Int Soc Sports Nutr. 14:20. Epub June 20, 2017. https://doi.orf/10.1186/s12970-017-0177-8
[5] Lacroix M., Bos C., Léonil J., Airinei G., Luengo C., Daré S., Benamouzig R., Fouillet H., Fauquant J., Tomé D., Gaudichon C. (2018). Compared with casein or total milk protein, digestion of milk soluble proteins is too rapid to sustain the anabolic postprandial amino acid requirement. Am J Clin Nutr 2006; 84:1070–9/ Epub January 23, 2018. https://doi.org/10.1556/1646.10.2018.10
[6] Ingredia. Prodiet 87 B Fluid – Nutrition facts . December 11, 2015.
[7] Ingredia. Prodiet 90 S – Nutrition facts . March 18, 2016.
[8] Res P.T., Groen B., Pennings B., Beelen M., Wallis G.A., Gijsen A.P., Senden J.M., Van Loon L.J. (2012) . Protein ingestion before sleep improves postexercise overnight recovery. Med Sci Sports Exerc 2012;44:1560–9. Epub January 2012. https://doi.org/10.1249/MSS.0b013e31824cc363
[9] Rasmussen C.J. (2008). Nutritional Supplements for Endurance Athletes. Nutritionnal supplements in sports and exercise, 369-407. https://doi.org/10.1007/978-1-59745-231-1_11
[10] Abbott W., Brett A., Cackburn E., Clifford T. (2019). Presleep casein protein ingestion: acceleration of functional recovery in professional soccer players. Int J Sports Physiol Perform. 14(3):385-391. Epub Feb 17, 2019. https://doi.org/10.1123/ijspp.2018-0385
[11] Snijders T., Res P.T., Smeets J.SJ., Van Vliet S., Van Kranenburg J., Maase K., Kies A.K., Verdijk L.B., Van Loon L.JC. (2015). Protein ingestion before sleep increases muscle mass and strength gains during prolonged resistance-type exercise training in healthy young men. The Journal of Nutrition, 145(6):1178-1184. Epub April 29, 2015. https://doi.org/10.3945/jn.114.208371
[12] Reitelseder S., Agergaard J., Doessing S., Helmark I.C., Lund P., Kristensen N.B., Frystyk J., Flyvbjerg A., Schjerling P., Van Hall G., Kjaer M., Holm L. (2011). Whey and casein labeled with L-[1-13C]leucine and muscle protein synthesis: effect of resistance exercise and protein ingestion. Am J Physiol Endocrinal Metab, 300, pp. E231-E242. Epub January 01, 2011. https://doi.org/10.1152/ajpendo.00513.2010
[13] Wilborn C.D., Taylor L.W., Outlaw J., Williams L., Campbell B., Foster C.A., Smith-Ryan A., Urbina S., Hayward S. (2013). The effects of pre- and postexercise whey vs. casein protein consumption on body composition and performance measures in collegiate female athletes. J Sports Sci Med 2013;12:74–9. Epub March 01, 2013. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3761774/
Why do endurance Athletes needs more protein?
Nowadays, athletes who practice resistance-type exercises (i.e. a short and intense sport such as fitness or powerlifting) have particular protein needs: these can be as high as two to three times the recommendation.
But endurance athletes (i.e. a moderate intensity sport over a relatively long period such as running, cycling, or natation) have also specific nutritional needs, particularly high-level athletes who sorely test their muscles!
During a long effort, proteins help to provide the energy needed by muscles. During recovery, they allow muscle to rebuild themselves and be ready for the next workout!
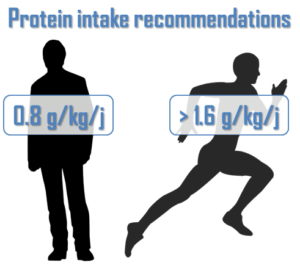
To understand it better, let’s consider an average person (non athlete). His body renews a part of its proteins everyday. A fraction is broken down, so there is an amino acids release which are then used to synthetize new proteins. This is the protein turnover (figure 1). However all amino acids from protein breakdown cannot be used for synthesis. A part of them are degraded then eliminated. Consequently, it is necessary to provide proteins to the body trough the diet to counterbalance these losses1. The protein needs of this person are about 0.8 g/kg of bodyweight per day2.
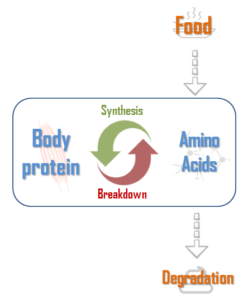
In this situation, there is a balance between proteins breakdown and synthesis, thus lean mass is maintained.
Protein synthesis is drastically reduced during a physical exercise in order to give the body’s energy resources to physical effort. During the first hours of exercise, the main energy source is glucose. When these carbohydrates tend to run out (after 2 hours), energy is provided by fat reserves. Proteins are used simultaneously, and they provide 2 to 8% of total energy: protein breakdown is increased3 4 5.
To draw up a summary: protein synthesis is reduced while breakdown is boosted. The protein balance is then negative.
Consuming proteins before training allows the body to have available free amino acids directly to satisfy its energy needs.
This thus makes it possible to limit the mobilization of muscle proteins. The quantity to consume depends on the exercise duration: the longer the training is, the more amino acids are oxidized to provide energy, so more proteins will be needed to compensate. It depends also on the last meal composition before the training: the more it is carbohydrate-rich, the more the glycogen reserved will be sufficient, therefore the less there will be necessary to mobilize alternative energy resources.5 6

After exercise, muscles are damaged. The body will regenerate them and rebuild in order to be well prepared for the next training: protein synthesis is increased (it is multiplied by 2 to 5 depending on the volume and the intensity of the exercise) during 24 to 72h6 7.
Simultaneously, protein breakdown remains high so much that if the person doesn’t increase his protein intake the global protein balance will remain negative8.
Consequently, it is recommended for athletes to consume proteins after training (a dose of approximately 20g). On one hand it will increase the blood concentration of essential amino acids, which are amino acids that body cannot synthetize. They must be supply by the diet. These ones have a robust anabolic effect: the muscle protein synthesis will be multiplied by 2 to 3 9. On the other hand it will increase the insulin blood concentration (even if there is no carbohydrate!) and this will strongly reduce the protein breakdown.6 9
Globally, these proteins taken just after training will stimulate the synthesis and reduce muscle protein breakdown: the overall protein balance is positive! This profile is favorable for muscle building, and thus performances improvement.
Finally, an intense and prolonged physical exercise is associated with numerous hormonal and biochemical changes within the body. This can have a negative impact on the immune system. In the case of proteins under-consumption, the restoration of the immune system will be limited. The person is therefore more inclined to contract an infection10.
To conclude, it is essential to consume more proteins, about twice the recommendation (which are only appropriate for an average person but not for a high-level athlete). It represents more than 1.6g/kg of bodyweight per day. This allows the body to maintain its integrity but also ensure the development of muscle mass and therefore performance improvement.4 10 11
So products for endurance athletes need to contain enough quality proteins. The chemical index is used to evaluate the nutritional quality of a protein. It is based on the essential amino acids amount. The reference is the FAO protein12, ideal since it contains all essential amino acids in minimal proportions necessary for body: its chemical index is set at 100.
However there are proteins with a chemical index higher than 100! For instance milk proteins, such as micellar caseins which have a chemical index of 128 13. These proteins are rich in essential amino acids.
These ones have a positive impact on muscle protein synthesis.
These milk proteins will be the subject of the next blog article!
For more information, please contact us
Authors: Rémi MALETERRE and Audrey BOULIER.
*Ironman triathlon: 2.4 miles (3.9km) of natation, 112 miles (180km) of cycling, 26.2 miles (42.2km) of running. For an athlete, it represents 7,000 to 10,000 kcal of energy consumption14.
_______________________________________________________
1. Poortmans, J.R., Carpentier, A., Pereira-Lancha, L.O., & Lancha Jr., A. (2012). Protein turnover, amino acid requirements and recommendations for athletes and active populations. Brazilian Journal of Medical and Biological Research, 45(10), 875-890. Epub June 06, 2012.https://dx.doi.org/10.1590/S0100-879X2012007500096
2. Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail (ANSES). (2019) Les protéines : définition, rôle dans l’organisme, sources alimentaires. Repéré à https://www.anses.fr/fr/content/les-prot%C3%A9ines
3. Moore D.R., Camera D.M., Areta J.L., Hawley J.A. (2014). Beyond muscle hypertrophy: why dietary protein is important for endurance athletes. Applied Physiology, Nutrition, and Metabolism, 39:987-997. Epub February 07, 2014. https://doi.org/10.1139/apnm-2013-0591
4. Tarnopolsky M., Gibala M., Jeukendrup A., Phillips S. (2007). Nutritional needs of elite endurance athletes. Part II: Dietary protein and the role of caffeine and creatine. Euro J Sport Sci, 2005;5(2):59-72. Epub February 20, 2007. https://doi.org/10.1080/17461390500137485
5. Rose A.J., Richter E.A. (2009). Regulatory mechanisms of skeletal muscle protein turnover during exercise. J Appl Physiol, 106:1702–11. Epub May 01, 2009. https://doi.org/10.1152/japplphysiol.91375.2008
6. Kumar V., Atherton P., Smith K., Rennie M.J. (2009). Human muscle protein synthesis and breakdown during and after exercise. J Appl Physiol 106, 2026–2039. Epub June 01, 2009. https://doi.org/10.1152/japplphysiol.91481.2008
7. Wilkinson S.B., Phillips S.M., Atherton P.J., Patel R., Yarasheski K.E., Tarnolpolsky M.A., Rennie M.J. (2008). Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 586: 3701–3717. Epub July 31, 2008. https://doi.org/10.1113/jphysiol.2008.153916
8. Phillips S.M., Tipton K.D., Aarsland A.A., Cortiella J.C., Wolf S.P., Wolfe R.R. (1997). Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am. J. Physiol. 273 (Endocrinol. Metab. 36): E99–E107. Epub July 01, 1997. https://doi.org/10.1152/ajpendo.1997.273.1.E99
9. Atherton P.J., Smith K. (2012). Muscle protein synthesis in response to nutrition and exercise. J Physiol, 590(5):1049–1057. Epub January 31, 2012. https://doi.org/10.1113/jphysiol.2011.225003
10. Gleeson M., Bishop N.C. (2000). Elite athlete immunology: importance of nutrition. Int J Sports Med, 21(Suppl 1):S44-50. 2000. https://www.thieme-connect.com/products/ejournals/html/10.1055/s-2000-1451#N68858
11. Phillips S.M., Van Loon L.J. (2011). Dietary protein for athletes: from requirements to optimum adaptation. J Sports Sci 29 (Suppl. 1), S29–38. Epub December 09, 2011. https://doi.org/10.1080/02640414.2011.619204
12. Report of an FAO Experte consultation. Dietary protein quality evaluation in human nutrition. ISSN 0254-4725 FAO and food nutrition paper 92. 31 March – 2 April, 2011.
13. Ingredia. Prodiet 87 B Fluid – Fiche nutritionnelle. 11 décembre 2015.
14. Triathlon inspires, Calories Burned during an Ironman Triathlon. Repéré à : http://www.triathloninspires.com/ti_fitness_and_health/calories/Calories_burned_IM.pdf
